Introduction to pH Meters
PH meters are an essential tool for all fields ranging from scientific laboratories to food and beverage to construction to farming. The acidity or alkalinity of a solution is an essential part of many biological and chemical reactions, and that is exactly what a digital pH meter measures.
Before a German physical chemist by the name of Walther Nernst came up with his namesake equation (more on that later), pH was measured with litmus paper, aka pH test strips, or liquid pH test kits.
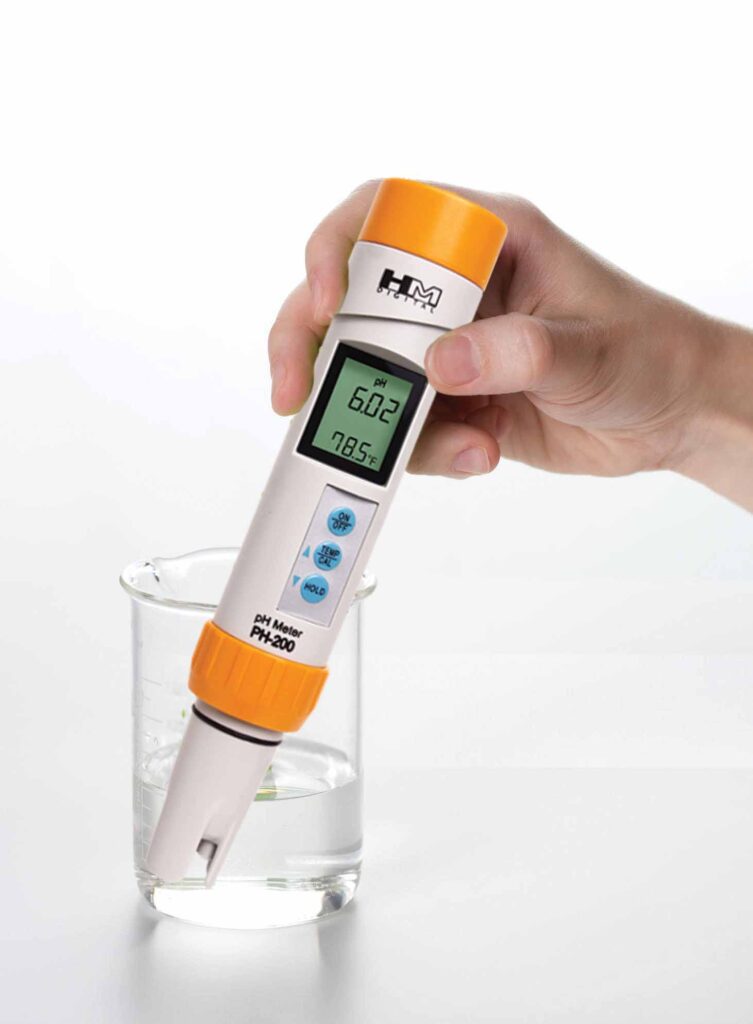
The idea is similar between the two, and you might even remember using them in science class or you might have used a liquid pH test kits to help balance a pool. Both change color after being exposed to the solution. And you just compare the color of the paper to the color chart that came with the strips to get a rough idea of the pH. Yup, we all measured pH with a chemical reaction on a piece of paper.
But that wasn’t good enough. Nope. We want faster readings, more accurate readings, and we want it now. Enter the digital pH meter which answered the call.
What is pH
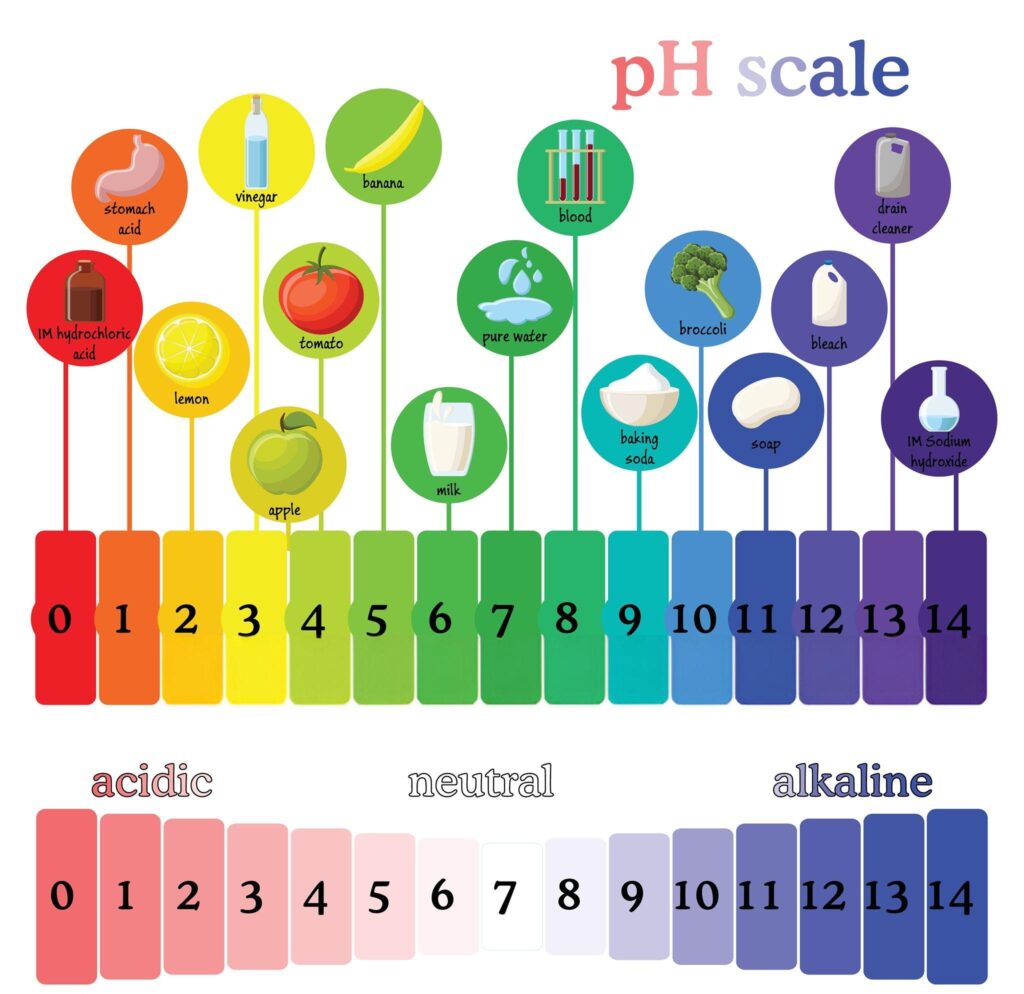
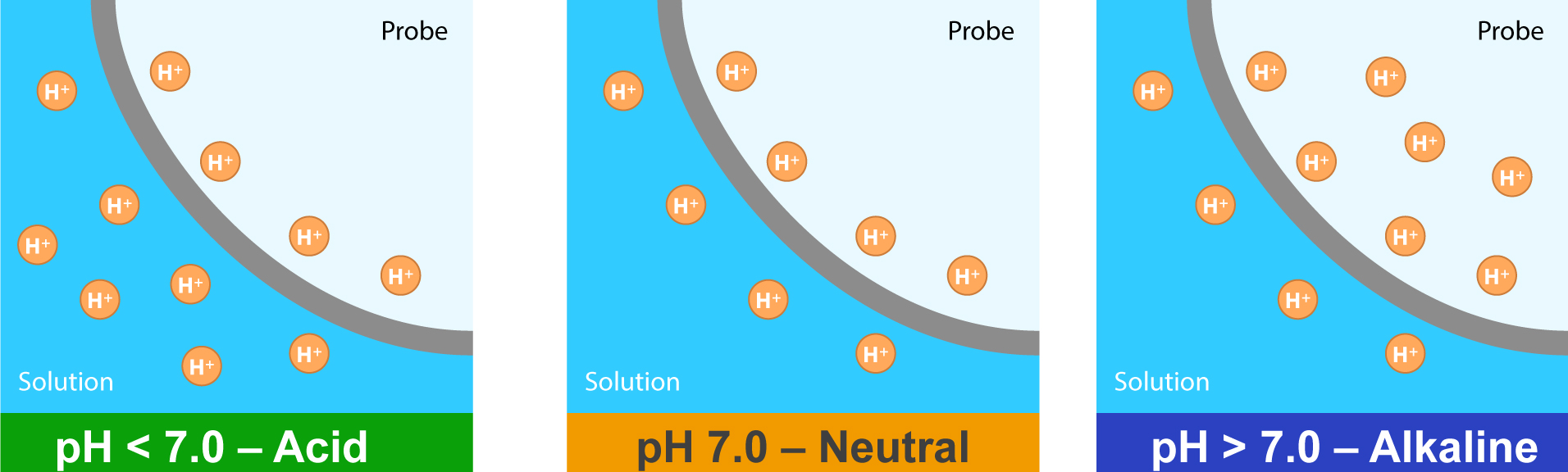
PH stands for “potential of Hydrogen”. Specifically, we’re measuring Hydrogen Ions (H+). PH is a term used to indicate the acidity or alkalinity of a substance, on a logarithmic scale of 0 to 14, pH = -log[H+],
You can see that pH relates directly to the amount of H+ (or H3O+).
A substance with a pH reading of 7.0 is a neutral solution, below 7 are acids, and above 7 are alkaline. The pH scale is logarithmic. Remember that the pH scale is logarithmic, so a pH of 5.0 is a magnitude more acidic than a pH of 6.0.
In pure distilled water, H+ and OH– are equal, so the pH is neutral (7.0)… It’s pretty simple: H+ + OH– = H20. So when levels of H+ are higher than OH–, you have an acidic solution, and when H+ is lower than OH–, you have an alkaline solution.
The Hydrogen Ion balance is important to remember later when I go over how the pH probe works.
Key Components of a pH Meter
The pH meter is a tool used to measure the pH of a solution, and it is comprised of several components.
The heart of any type of pH meter is the pH probe. This essential device is submerged in the solution to measure pH levels. It contains a special glass electrode, typically shaped like a bulb, filled with a buffer solution of known pH. In the case of a pH pen, the probe is attached directly to the body. In other, handheld pH meters the probe is at the end of a cable.
Besides the glass electrode, the most good quality pH meters contain a temperature sensor to compensate for temperature variations that affect the glass’ reactivity, to produce more precise readings regardless of the solution being tested.
There is also the pH meter body itself that usually includes replaceable batteries. The body will act as the “brains” of the pH meter and includes a digital screen and several buttons to control the meter itself.
The Indicator Electrode and the Reference Electrode
The reference electrode is a very important part in every pH sensor. It provides a voltage reading so the meter can measure the voltage difference between the reference electrode and the indicator electrode, allowing the pH meter to accurately determine the pH value. Essentially, it serves as a stable point of comparison for the indicator electrode’s potential, providing accurate pH readings.
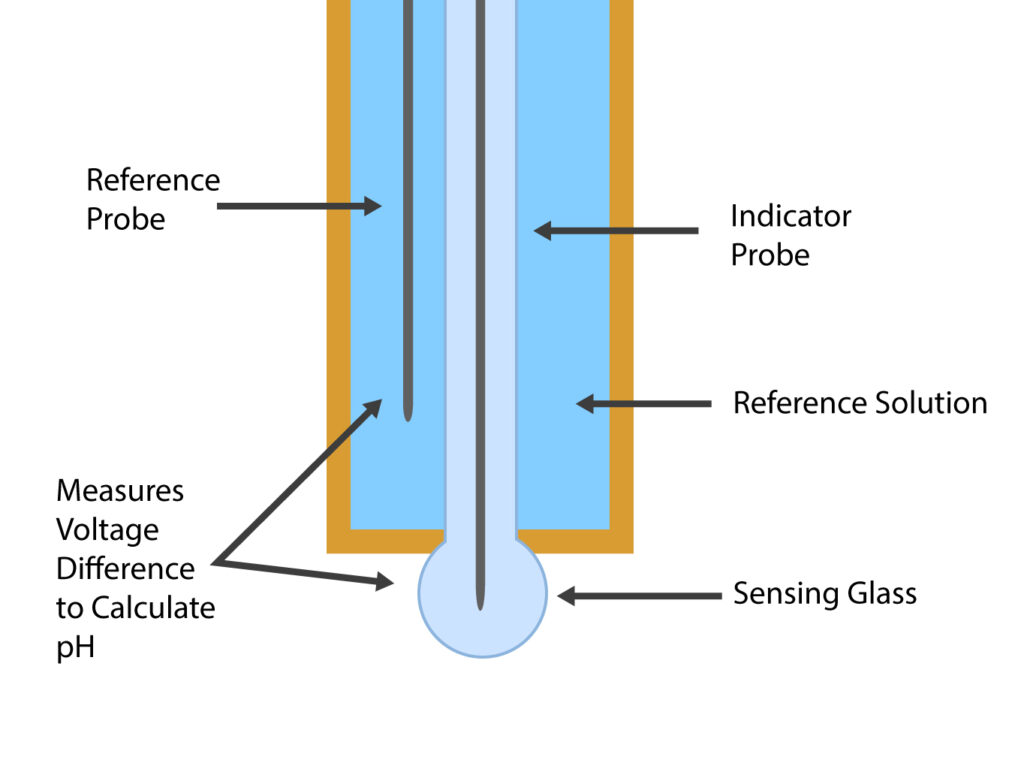
Understanding how the reference electrode functions is key to appreciating its importance. During a pH test, the test solution contacts a known solution through a porous plug at the bottom of the reference electrode.
The reference electrode is immersed in a solution (usually Potassium Chloride, KCl) with a known concentration of ions that maintain a stable pH, even with external factors like temperature changes. Hydrogen Ions flow from the reference electrode solution into the test solution to produce the electrical charge.
Voltage is generated on the surface of the sensing glass. If the voltage is positive, then the test solution is acidic, and if the voltage is negative, the test solution is alkaline.
The Meter Body or Display Unit
The digital display is an essential part of the pH meter, translating the electrode’s measurements into a readable format. On a well maintained pH meter the digital display is so much better than litmus paper. You get pH measurements in decimal format, compensated for temperature, and a temperature reading too.
The body will also have some buttons that will allow you to change display settings and control the calibration process.
Every pH meter has it’s own calibration process, but essentially, you choose two of three available buffer solutions: 4.0, 7.0, and 10.0. Most farmers are measuring acidic solutions, so they will calibrate their pH meter using 4.0 and 7.0 buffer solutions because they cover their desired range of the nutrient solutions they feed their plants.
When the pH meter calibrates, it is basically adjusting the setting for those calibration points so the meter can provide accurate results.
The Science Behind pH Meters
A pH meter actually just measures the electrical potential in a solution. An acidic solution contains a higher concentration of positively charged Hydrogen ions than an alkaline solution. Hydrogen ions conduct electricity, so an acidic solution will conduct more of an electrical current than an alkaline solution.
How does a pH meter work?
One of the main components of a pH meter is the glass electrode or pH probe.
The electrode is constructed of a special glass with a reference electrolyte inside, usually potassium chloride solution, surrounding the electrodes. There can be two or three separate chambers, also called single or dual junction probes.
When you place a pH probe into a solution, hydrogen ions move toward the glass electrode, replacing some metal ions inside. Some hydrogen ions also disperse into the solution. This is called ion exchange and is how the glass electrode in the pH probe functions.
Ion exchange also happens on the inner surface of the glass electrode. The different acidity levels between the potassium chloride inside the electrode and the solution lead to different hydrogen ion activities, causing a charge difference.
This results in a potential difference between the sides of the glass electrode and the reference electrode, producing a pH reading on the meter. The meter measures the voltage, but the digital display shows it as a pH value, calculated based on the voltage difference.
Greater voltage between the pH probe and the solution indicates a higher hydrogen ion activity, leading to a lower pH value, and vice versa. Remember the formula above is an inverse scale which is the same as: pH = -1 x log[H+]
The Nernst equation helps predict the potential measured by a pH meter based on hydrogen ion concentration. Named after Walter Nernst, this equation shows that the potential difference across the glass electrode is related to the hydrogen ion activity. Thanks to Nernst’s work, pH meters can accurately read pH levels in solutions.
Factors that Can Impact the Accuracy of pH Meters
There are a few factors that can help you optimize the performance and extend the life of your digital pH meter.
One of the biggest factors that affects pH Meters is called electrode drift. It occurs when a probe becomes less sensitive to solutions over time resulting in consistently higher or lower readings than the actual pH of the solution. This happens over time to all probes because of impurities in the solution, age of the probe, contamination in the potassium chloride solution inside the probe, and contaminants on the surface of the glass.
Otherwise the accuracy of the meter usually depends on physical factors like temperature and solution purity.
Temperature impacts chemical reactions, including those measured by pH meters. Variations can change the hydrogen ion concentration, so controlling temperature is extremely important for accurate readings.
Impurities in the solution can also affect accuracy by interacting with hydrogen ions.
Finally, the age of the pH probe plays a massive role. Over time, all probes deteriorate, reducing its measurement accuracy. You can slow that process down by rinsing the probe in deionized water or distilled water, after each use. Then, using storage solution.
Regular maintenance and timely replacements are crucial to make sure your pH meter produces precise measurements.
Maintenance and Safe Use of pH Meters
Keeping your pH meter in tip-top shape is absolutely essential for accurate measurements. It all begins with the calibration process, which means comparing your meter’s readings with known buffer solutions. Better pH meters will calibrate with two buffers that cover the expected pH range of your samples.
Besides regular calibrations, you need to take care of the pH probe itself, it is the main component that interacts with your samples. After each use, rinse the electrode gently to remove any leftover solution and prevent contamination of the probe. Don’t touch it or wipe it, as this can damage the probe or create static electricity and mess up its sensitivity. A gentle rinse with distilled or de-ionized water is the best way to clean it.
When you’re not using the pH meter, store the probe in a little bit of storage solution. Most pH electrodes should be stored in a special storage solution recommended by the manufacturer to keep the glass sensor hydrated. Don’t store it in distilled or deionized water! Doing that can draw the ions out of the electrode’s solution and damage it over time.
And that’s where I’ll leave off — make sure you maintain your probe. It is a bit of a chore, but regularly rinsing and using storage solution will allo you to get more use out of your digital pH meter and you’ll get more accurate readings over time.
References
- PH Measurement and Uses, Community College of Rhode Island.
- PH Meter – Instrument, Iowa State University.
- Nernst Equation, Wikipedia
- What causes errors in pH sensor readings, Icon Process Controls
- PH Probe Accuracy, NBCHAO Lab Testing Instrument